The Best Longevity Supplements: A No-Hype, Science-Based Guide

By Brent | Last Updated: February 3rd, 2026
The Best Longevity Supplements: A No-Hype, Science-Based Guide
Featured Snippet Candidate: Longevity supplements are compounds designed to target the biological mechanisms of aging, such as mitochondrial decline, chronic inflammation, and cellular senescence, rather than just addressing nutrient deficiencies. The most researched options include NAD+ precursors (NMN and NR), spermidine, CoQ10, and fisetin. While animal studies are promising, no supplement has yet been proven to extend human lifespan, and they work best alongside, not instead of, healthy lifestyle habits.
Key Takeaways
> Longevity supplements target aging pathways like inflammation, mitochondrial dysfunction, and senescent cell buildup, not just vitamin gaps.
> Most evidence comes from animal studies and early human trials. Definitive proof of lifespan extension in humans doesn't exist yet.
> Supplements are tools, not magic. They support a foundation of sleep, movement, nutrition, and stress management, they don't replace it.
> Quality matters enormously. The supplement industry is poorly regulated, and many products don't contain what they claim.
> Testing and tracking (blood panels, biological age tests) help you know if a supplement is actually doing anything for you.
What Are Longevity Supplements, Exactly?
Most supplements you'll find at the pharmacy are designed to fill nutritional gaps, vitamin D if you're deficient, iron if you're anemic, and so on.
Longevity supplements are different. They're compounds intended to slow biological aging itself by targeting the underlying mechanisms that cause us to deteriorate over time.
Citation-Worthy: Longevity supplements aim to influence the hallmarks of aging, processes like mitochondrial dysfunction, chronic low-grade inflammation, impaired autophagy, and the accumulation of senescent "zombie" cells, rather than simply correcting deficiencies.
Think of it this way: standard supplements patch holes. Longevity supplements attempt to slow down the wear and tear that creates the holes in the first place.
That said, let's be clear upfront: no longevity supplement has been proven to extend human lifespan. The research is promising, the biology is plausible, but we're still in the early innings. Anyone who tells you otherwise is selling something.
How Aging Works (The 60-Second Version)
To understand why certain supplements get longevity researchers excited, it helps to know what's actually happening as we age.
Scientists have identified several "hallmarks of aging", shared biological processes that drive decline across nearly all tissues and organ systems. The major ones include:
> Mitochondrial dysfunction: Your mitochondria (the power plants inside your cells) become less efficient at producing energy and generate more damaging byproducts.
> Chronic inflammation: Low-grade, persistent inflammation, sometimes called "inflammaging," accelerates tissue damage and disease risk.
> Impaired autophagy: Autophagy is your body's cellular cleanup system. When it slows down, damaged proteins and organelles accumulate.
> Cellular senescence: Some damaged cells stop dividing but don't die. These "zombie cells" secrete inflammatory signals that harm neighboring healthy cells.
> Telomere attrition: The protective caps on your chromosomes shorten with each cell division, eventually limiting cellular replication.
> NAD+ decline: Levels of NAD+, a molecule essential for energy metabolism and DNA repair, drop significantly with age.
Longevity supplements attempt to address one or more of these pathways. Some boost NAD+ levels. Others activate autophagy. A few may help clear senescent cells.
The catch? Most of this research has been done in yeast, worms, mice, or cell cultures. Humans are more complicated, and what works in a lab mouse doesn't always translate to a 55-year-old human.
Supplements vs. Prescription Drugs: Know the Difference
Before we go further, an important distinction:
Supplements are not regulated by the FDA the same way drugs are. They don't require large-scale human trials to prove safety or efficacy before hitting shelves. Manufacturers are responsible for their own quality control, which varies wildly.
Prescription drugs like metformin and rapamycin are sometimes discussed in longevity circles, but they're not supplements. They require a doctor's prescription, have known side effects, and are being studied in formal clinical trials for aging-related applications.
We'll touch on metformin and rapamycin later, but understand: taking a prescription drug off-label for longevity is a very different decision than adding a supplement to your morning routine. One requires medical supervision; the other, you can order on Amazon (for better or worse).
Why Consider Longevity Supplements?
Fair question. If the evidence is still emerging, why bother?
A few reasons people take the plunge:
> Proactive health investment. Treating disease is expensive, financially and physically. Some people prefer to invest modestly now in strategies that might reduce risk later.
> Lifestyle optimization. If you're already sleeping well, exercising, eating right, and managing stress, supplements can be the next lever to pull.
> Personalized experimentation. With tools like blood panels and biological age tests, you can actually measure whether a supplement is moving the needle for you specifically.
> Hedging bets. The downside of most well-researched supplements (taken responsibly) is relatively low. The potential upside, if the science pans out, could be meaningful.
That said, supplements are never a substitute for the basics. If you're sleeping five hours a night and living on processed food, a pill isn't going to save you. Fix the foundation first.
The Top Longevity Supplements, Ranked by Evidence
Let's get into the specifics. Below are the most commonly discussed longevity supplements, organized roughly by the strength of current research and biological plausibility.
Important caveat: "More evidence" doesn't mean "proven to extend lifespan." It means the compound has been studied more extensively, shows consistent effects on relevant biomarkers, and has a plausible mechanism. Your mileage may vary.
NAD+ Precursors (NMN and NR)
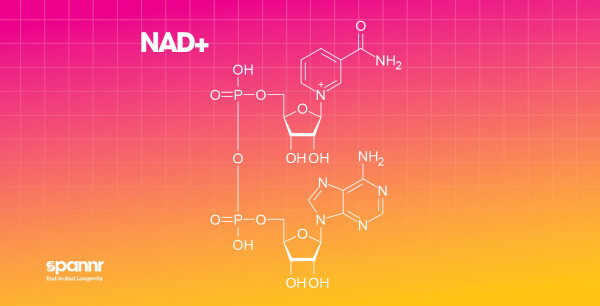
What they are: NMN (nicotinamide mononucleotide) and NR (nicotinamide riboside) are precursors to NAD+, a coenzyme involved in hundreds of metabolic processes including energy production, DNA repair, and sirtuin activation.
Why they matter: NAD+ levels decline substantially with age, by some estimates, dropping 50% or more between your 40s and 60s. This decline is linked to mitochondrial dysfunction, impaired DNA repair, and increased disease risk.
What the research shows: Animal studies consistently show that boosting NAD+ improves metabolic health, muscle function, and in some cases, lifespan. Human trials are smaller but have shown improvements in markers like insulin sensitivity and muscle function, particularly in older adults.
Citation-Worthy: Human studies on NMN and NR have demonstrated improvements in metabolic markers and muscle function, though long-term effects on lifespan remain unproven.
The fine print: NMN's regulatory status has gotten complicated. The FDA has indicated it may be investigated as a drug, which could affect supplement availability. Quality varies significantly between brands. Typical doses in studies range from 250mg to 1,000mg daily for NMN.
Bottom line: Among the most promising longevity supplements based on mechanistic plausibility and emerging human data. Not proven, but biologically compelling.
Spermidine
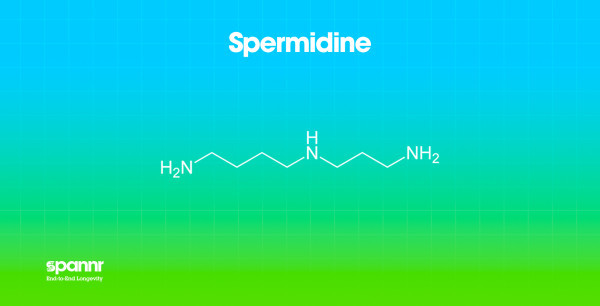
What it is: A naturally occurring polyamine found in foods like aged cheese, mushrooms, soybeans, and wheat germ. It's also produced by gut bacteria.
Why it matters: Spermidine is one of the most potent known activators of autophagy, the cellular cleanup process that removes damaged proteins and organelles.
What the research shows: Observational studies have linked higher dietary spermidine intake to reduced cardiovascular mortality and improved cognitive function in older adults. Animal studies show extended lifespan in multiple species.
Citation-Worthy: Epidemiological research suggests that higher spermidine intake is associated with reduced mortality risk, making it one of the more promising dietary longevity compounds currently under investigation.
The fine print: Most human evidence is observational (people who eat more spermidine-rich foods tend to live longer), which doesn't prove causation. Randomized controlled trials are ongoing. Typical supplement doses range from 1-6mg daily.
Bottom line: Strong biological rationale, supportive observational data, and a good safety profile. One of the more interesting options for autophagy support.
Coenzyme Q10 (CoQ10)
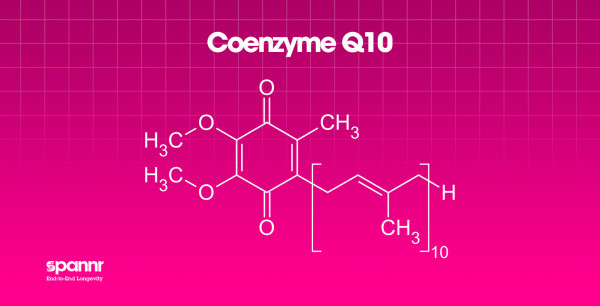
What it is: A compound your body produces naturally that plays a central role in mitochondrial energy production and acts as an antioxidant.
Why it matters: CoQ10 levels decline with age, and lower levels have been associated with cardiovascular disease, neurodegenerative conditions, and general fatigue.
What the research shows: Supplementation has been shown to improve symptoms in heart failure patients, reduce oxidative stress markers, and may support mitochondrial function in older adults. The evidence for direct longevity effects is indirect but mechanistically sound.
The fine print: Ubiquinol (the reduced form) is generally better absorbed than ubiquinone. Typical doses range from 100-300mg daily. CoQ10 is fat-soluble, so take it with a meal containing fat.
Bottom line: Well-established for mitochondrial and cardiovascular support. A reasonable choice for adults over 50, especially those on statins (which deplete CoQ10).
Fisetin
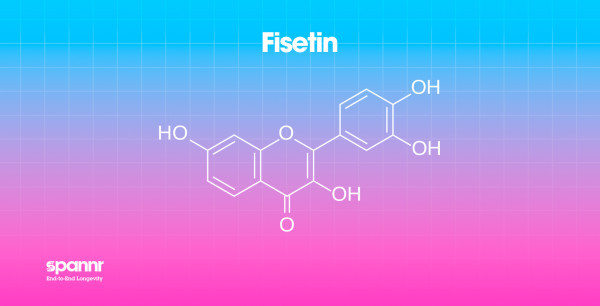
What it is: A flavonoid found in strawberries, apples, persimmons, and onions. It's gained attention as a potential senolytic, a compound that may help clear senescent cells.
Why it matters: Senescent cells accumulate with age and secrete inflammatory signals (the "senescence-associated secretory phenotype" or SASP) that damage surrounding tissue. Clearing these cells, in theory, could reduce inflammation and slow aging.
What the research shows: Animal studies show fisetin can reduce senescent cell burden, improve physical function, and extend lifespan in mice. Human trials are underway but results are still emerging.
The fine print: Optimal dosing is unclear. Some researchers suggest intermittent high-dose protocols (mimicking how senolytics work in animal studies) rather than daily low doses. Bioavailability is a challenge.
Bottom line: Exciting mechanism with strong animal data. Human evidence is still early. Worth watching, but probably not a first-line choice yet.
Quercetin
What it is: Another flavonoid, found in onions, apples, berries, and capers. Often discussed alongside fisetin as a potential senolytic.
Why it matters: Quercetin has antioxidant and anti-inflammatory properties, and some research suggests it may support immune function and NAD+ metabolism.
What the research shows: In combination with dasatinib (a prescription cancer drug), quercetin has shown senolytic effects in human studies. However, quercetin alone may not reliably clear senescent cells based on cell culture studies.
The fine print: The senolytic hype around quercetin may be overstated when used as a standalone supplement. Benefits likely depend on context, dose, and combination with other compounds.
Bottom line: Useful as an antioxidant and anti-inflammatory. The senolytic angle is interesting but probably requires combination therapy to be meaningful.
Curcumin
What it is: The primary active compound in turmeric, responsible for its yellow color and many of its biological effects.
Why it matters: Chronic low-grade inflammation is a major driver of aging. Curcumin has potent anti-inflammatory and antioxidant properties, and may influence pathways like mTOR and NF-κB.
What the research shows: Studies show curcumin can reduce inflammatory markers like CRP and IL-6, and may offer benefits for joint health, cognitive function, and cardiovascular risk factors.
The fine print: Bioavailability is notoriously poor. Standard turmeric powder delivers very little active curcumin to your bloodstream. Look for formulations with piperine (black pepper extract) or lipid-based delivery systems that enhance absorption.
Bottom line: A solid anti-inflammatory with good safety data. Choose a bioavailable formulation or you're mostly wasting money.
Resveratrol
What it is: A polyphenol found in red wine, grapes, and berries. It became famous after studies showed it extended lifespan in yeast and some animal models.
Why it matters: Resveratrol activates sirtuins (proteins involved in cellular stress response and longevity pathways) and influences mitochondrial function and oxidative stress.
What the research shows: The animal data was exciting. The human data has been... disappointing. Multiple trials have failed to show consistent benefits for metabolic health or cardiovascular risk factors in humans.
The fine print: Bioavailability is low, and the doses that worked in mice would translate to impractically large amounts in humans. The "red wine is good for longevity" narrative has largely been debunked, the resveratrol content in wine is too low to have meaningful effects.
Bottom line: Mechanistically interesting, but human evidence is weak. Not a top-tier choice based on current data.
What About Metformin and Rapamycin?
You'll hear these names in longevity circles constantly. Let's clarify:
Metformin is a diabetes drug that's being studied in the TAME (Targeting Aging with Metformin) trial for its potential to delay age-related diseases. It's relatively safe, inexpensive, and some longevity-focused physicians prescribe it off-label. But it's a prescription medication, not a supplement.
Rapamycin is an immunosuppressant that has extended lifespan in nearly every organism tested. It works by inhibiting mTOR, a growth pathway implicated in aging. Some doctors prescribe low-dose, intermittent rapamycin for longevity purposes, but this is experimental and carries risks including immune suppression.
Both require medical supervision and ongoing monitoring. They're not Amazon purchases.
How to Avoid Getting Scammed
The longevity supplement market is a minefield. Here's how to navigate it:
Look for third-party testing. Brands that submit products to independent labs (NSF, USP, ConsumerLab) and publish results are more trustworthy than those that don't.
Check for transparent labeling. You should know exactly what's in the bottle and in what amounts. Avoid "proprietary blends" that hide dosages.
Be skeptical of miracle claims. If a product promises to "reverse aging" or "add 10 years to your life," run. No supplement can deliver that.
Research the company. How long have they been around? Do they have scientists on staff or an advisory board? Do they cite studies to support their claims?
Start with one thing at a time. If you stack five supplements at once, you'll have no idea which one is helping (or causing side effects).
Testing: How to Know If It's Working
Here's the unsexy truth: without testing, supplementation is guesswork.
Blood panels can reveal changes in inflammation markers, metabolic health, and nutrient levels. Biological age tests (like TruAge or GrimAge) attempt to estimate how fast you're aging at a cellular level.
If you take a supplement for six months and your markers don't improve, or they get worse, that's useful information.
Consider working with a longevity-focused physician or health coach who can help you design a testing protocol and interpret results.
Who Should (and Shouldn't) Consider Longevity Supplements
Good candidates:
> Adults 40+ who have already optimized sleep, exercise, and nutrition
> People using biomarker testing to guide decisions
> Those with a long-term, patient mindset (not looking for quick fixes)
Not good candidates:
> Anyone expecting supplements to compensate for poor lifestyle choices
> People under 40 without specific health concerns (focus on the basics first)
> Those who can't afford quality products or testing
How to Start: A Practical Approach
If you're new to longevity supplements, here's a sensible path:
> Get baseline testing. Blood panels, and ideally a biological age test. Know where you're starting.
> Fix deficiencies first. Low vitamin D? Address that before chasing NAD+.
> Choose one primary compound. Based on your health goals and the evidence, pick one supplement to try for 3-6 months.
> Track and reassess. Retest after a reasonable period. Did anything improve? Any side effects?
> Adjust accordingly. If it's working, continue. If not, try something else or accept that supplements may not be your highest-leverage intervention.
Minimalism beats maximalism here. A carefully chosen, high-quality supplement is more valuable than a kitchen counter full of bottles.
Final Perspective
Longevity supplements are tools, potentially useful ones, but tools nonetheless. They're not shortcuts, not magic pills, and not substitutes for the boring-but-effective fundamentals of healthy aging.
The science is genuinely exciting. Compounds like NMN, spermidine, and fisetin have real biological plausibility and growing research support. But we're still early in understanding how these translate to longer, healthier human lives.
In 2026, the smartest approach remains: move your body regularly, eat mostly whole foods, sleep like it matters (because it does), manage your stress, stay socially connected, and then, if you want to experiment with supplements, do so thoughtfully, with testing, and with realistic expectations.
That's not as sexy as a miracle pill. But it's honest. And it's the approach most likely to actually help you age well.
FAQs about Longevity Supplements
What is the best supplement for longevity?
There's no single "best" option, it depends on your biology, health status, and goals. That said, NAD+ precursors (NMN or NR) and spermidine currently have the strongest combination of mechanistic plausibility and emerging human evidence. Start with one, test your response, and go from there.
Do longevity supplements actually work?
They can influence biological pathways associated with aging, and some show improvements in biomarkers like inflammation, metabolic health, and cellular function. However, no supplement has been proven to extend human lifespan. Think of them as supporting tools, not guarantees.
Are longevity supplements safe?
Most well-researched longevity supplements are safe at appropriate doses for healthy adults. However, long-term effects aren't fully known, and interactions with medications are possible. Start low, monitor how you feel, and consult a healthcare provider if you have existing conditions.
How long does it take to see results from longevity supplements?
It varies by compound and what you're measuring. Some people notice subjective improvements (energy, sleep quality) within weeks. Biomarker changes typically require 3-6 months of consistent use to assess. Patience is part of the process.
Should I take longevity supplements if I'm under 40?
For most people under 40, lifestyle optimization (sleep, exercise, nutrition, stress management) will deliver far more benefit than supplements. Exceptions might include addressing specific deficiencies or health concerns. Don't skip the fundamentals to chase pills.
What supplements does Bryan Johnson take?
Bryan Johnson follows an extensive, highly personalized protocol called Blueprint that includes dozens of supplements alongside strict diet, exercise, sleep, and medical monitoring. His approach is extreme, expensive, and not generalizable to most people. It's interesting to follow, but not a template for the average person.
Can I combine multiple longevity supplements?
You can, but more isn't necessarily better. Stacking many compounds increases cost, complexity, and the risk of interactions. A more effective approach: start with one, assess its impact, and only add others if there's a clear rationale.
About the Author
Sign Up For Our Newsletter
Weekly insights into the future of longevity
